Disease area
Non-alcoholic fatty liver disease is the hepatic manifestation of metabolic disorder and is outlined by hepatic lipid accumulation that may progress to an aggressive form of fatty liver disease: the non-alcoholic steatohepatitis (NASH). NASH is characterized by liver inflammation, progressive scarring and fibrosis causing cirrhosis in most of the cases thus leading to liver failure (1). Currently, there is no approved pharmacological treatments for NASH (2), thus, screening the pre-annotated donated chemical probe (DCP) set with relevant phenotypic endpoints could shed light on the disease pathophysiology and would enable identification of druggable targets.
Rationale
One of the key limitations in drug development for NASH is the unattainability of an in vitro model that recapitulates faithfully the various stages of the disease and their major hallmarks. For this purpose, we find it crucial to develop a NASH model that truly depicts all aspects of the disease.
Aim
We have previously established a screening platform to assess compounds effects on steatosis, inflammation, and fibrosis in our patient-derived NASH in vitro model. This data sheet elaborates on DNA damage response proxied by nuclear 53BP1 expression (3) as another interesting endpoint relevant to NASH pathophysiology.
Methods
Cell culture: We co-cultured patient-derived non-parenchymal cells (NPC, LifeNet) from a donor with early-stage fibrosis with healthy primary human hepatocytes (PHH, BioIVT) (6:1 PHH to NPC ratio) in a multi-well 96-well plate format (Elplasia, Corning). We seeded the cells at a density of 40,000 cells/well in William’s E medium supplemented with 11mM glucose, insulin (10ng/mL in Control or 10μg/mL in NASH), 5.5μg/mL transferrin, 6.7ng/mL selenite, 100nM dexamethasone, 2mM L-glutamine, 100U/mL penicillin, 100μg/mL streptomycin, and 10% fetal bovine serum (FBS).
Upon spheroid aggregation (5-7 days), we phased out FBS; to aggravate NASH phenotype we added a 0.6% (w/v) albumin-conjugated free fatty acid (FFA) into the media, consisting of equimolar palmitic and oleic acid (240μM final concentration each). FFA was not supplemented in Control, mimicking lifestyle intervention by reducing nutrition and fat uptake. For the treatment groups, we treated the spheroids with FFA and 10μM TH10785 for 1 week with redosing every 2-3 days.
Read-out:
DNA damage:
Spheroids were collected, fixed with 4% formaldehyde for 24 h, cryoprotected in 30% sucrose and embedded in Tissue-Tek OCT compound (Sakura, The Netherlands). Spheroid cryosections (8 μm) were stained for 53BP1 (Abcam, ab36823, 1/1000 dilution) and then counter-stained with DAPI in mounting media (Invitrogen, P36935). Images were obtained with 63x objective magnification (oil immersion) with Leica Stellaris 5 LIA. Images were analysed with Fiji (ImageJ) to identify and count 53BP1 foci and nuclei (see Figure 1).
Results
DNA damage: Modulation of OGG1 activity reduced the number of 53BP1 foci. These results indicate that the burden of DNA double-strand breaks is reduced. Results for the OGG1 modulator TH10785 are shown in figure 1.
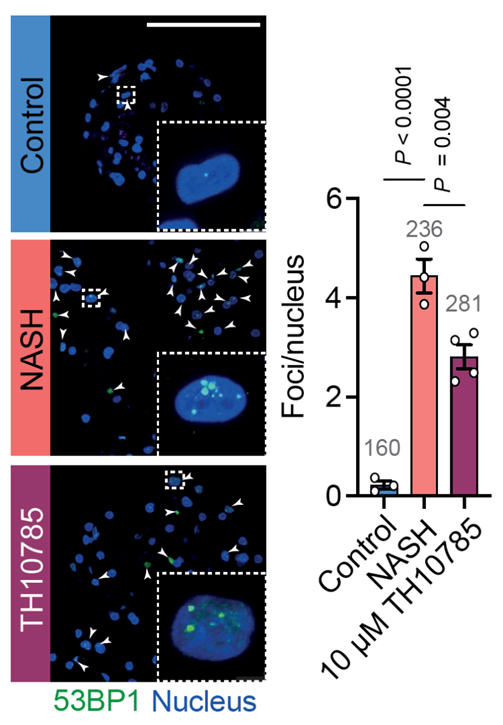
Figure 1: TH10785 resulted in a significant reduction of 53BP1. Insets show a representative nucleus at higher magnification. 53BP1 foci were quantified and normalized across nuclei (the number of analyzed nuclei is shown in grey on top of the bars). Scale bar = 100 μm. T-test performed for each group against NASH (P-values shown on top).
Conclusions
FFA supplementation increased the double-strand DNA damage burden in our in vitro NASH model and TH10785 treatment showed improved DNA damage repair.
References
- Schwabe R et al.; Mechanisms of Fibrosis Development in NASH (2020).
- Kemas et al.; Non-alcoholic fatty liver disease - opportunities for personalized treatment and drug development (2021).
- Panier, S. and Boulton, S. Double-strand break repair: 53BP1 comes into focus (2013).



