Disease area
Non-alcoholic fatty liver disease is the hepatic manifestation of metabolic disorder and is outlined by hepatic lipid accumulation that may progress to an aggressive form of fatty liver disease: the non-alcoholic steatohepatitis (NASH). NASH is characterized by liver inflammation, progressive scarring and fibrosis causing cirrhosis in most of the cases thus leading to liver failure (1). Currently, there is no approved pharmacological treatments for NASH (2), thus, screening of chemical series using relevant phenotypic endpoints could shed light on the disease pathophysiology and would enable identification of druggable targets.
Rationale
One of the key limitations in drug development for NASH is the unattainability of an in vitro model that recapitulates faithfully the various stages of the disease and their major hallmarks. For this purpose, we find it crucial to develop a NASH model that truly depicts all aspects of the disease.
Aim
We want to develop a patient-derived NASH model that manifests the key characteristics of the disease namely steatosis, inflammation, and fibrosis. Afterwards, we will use this model as a platform to screen compound panels and evaluate their impact on NASH progression by measuring intracellular lipid accumulation, inflammatory markers as well as fibrosis precursors.
Methods
Cell culture: Primary human hepatocytes (PHH) were thawed and seeded in hepatocyte culture medium (Williams E medium with 2 mM L-glutamine, 100 units/ml penicillin, 100 μ g/ml streptomycin, 10 μ g/ml insulin, 5.5 μ g/ml transferrin, 6.7 ng/ml sodium selenite, 100 nM dexamethasone) as previously described for PHH at 1,500 cells/well (3). Cells aggregated over the course of 5–7 days into one single spheroid per well. During this time, the aggregates compacted and, once fully formed, spheroid sizes pivoted around 200 μm. Once spheroids were formed, exposures were started in FBS-free hepatocyte culture medium containing compounds or vehicle. PHH spheroids were then exposed for 5 days after spheroid formation to a medium containing 5 μM A- 8301 and 3 μM CHIR99021 plus 1 μM of the respective chemical probe.
Read-out:
Hepatocyte proliferation: Hepatocytes undergoing proliferation were identified by revealing S-phase. To this end, 10 μM EdU (Sigma Aldrich/Merck) was added into the culture medium and revealed onto fixed spheroids by incubation for 30 minutes in staining solution (0.1 M Tris pH 7.5, 2 mM CuSO4, 5 μM Alexa 647-azide (Thermo Fisher), 100 mM ascorbic acid). Nuclei were counterstained using DAPI (Thermo Fisher). Imaging of whole-mount spheroids was performed in culture plates using an Opera Phenix High-Content Screening System (PerkinElmer).
Results
Hepatocyte proliferation: In total, 25 of the 108 probes significantly altered human hepatocyte proliferation after the Benjamini-Hochberg correction. These results suggest that epigenetic plasticity constitutes an essential prerequisite for hepatocyte proliferation. Results for epigenetic probes are shown in figure 1 (modified from ref. 4).
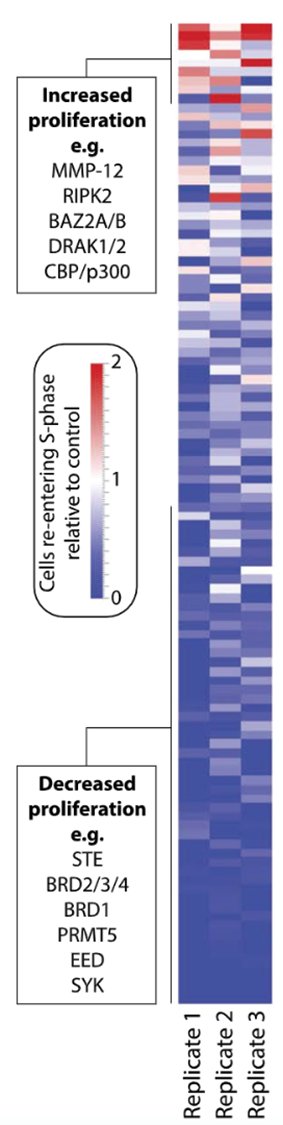
Figure 1: Chemogenomic screen reveals the importance of epigenetic plasticity for human hepatocyte regeneration. Heatmap representation of EdU quantification data of all 108 tested probes in biological triplicates.
Conclusions
We developed a patient-derived assay for NASH that can faithfully reproduce the hallmarks of the disease. We used this assay to screen for potential drug targets using the donated chemical probe (DCP) compound set. As a result of this screening, we have identified probes that efficaciously reduced steatosis revealing potential drug targets for NASH. Additionally, we validated the specificity of the hit probes by using non-active analogues and by showing a dose-dependent effect (data not shown).
References
- Schwabe R et al.; Mechanisms of Fibrosis Development in NASH (2020).
- Kemas et al.; Non-alcoholic fatty liver disease - opportunities for personalized treatment and drug development (2021).
- Bell et al.; Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease (2016).
- Oliva-Vilarnau et al.; Wnt/β-catenin and NFκB signaling synergize to trigger growth factor-free regeneration of adult primary human hepatocytes (2023).



