Disease area
Non-alcoholic fatty liver disease is the hepatic manifestation of metabolic disorder and is outlined by hepatic lipid accumulation that may progress to an aggressive form of fatty liver disease: the non-alcoholic steatohepatitis (NASH). NASH is characterized by liver inflammation, progressive scarring and fibrosis causing cirrhosis in most of the cases thus leading to liver failure (1). Currently, there is no approved pharmacological treatments for NASH (2), thus, screening the pre-annotated donated chemical probe (DCP) set with relevant phenotypic endpoints could shed light on the disease pathophysiology and would enable identification of druggable targets.
Rationale
One of the key limitations in drug development for NASH is the unattainability of an in vitro model that recapitulates faithfully the various stages of the disease and their major hallmarks. For this purpose, we find it crucial to develop a NASH model that truly depicts all aspects of the disease.
Aim
We want to develop a patient-derived NASH model that manifests the key characteristics of the disease namely steatosis, inflammation, and fibrosis. Afterwards, we will use this model as a platform to screen the DCP compounds and evaluate their impact on NASH progression by measuring intracellular lipid accumulation, inflammatory markers as well as fibrosis precursors.
Methods
Cell culture: We co-cultured patient-derived non-parenchymal cells (NPC, LifeNet) from a donor with early-stage fibrosis with healthy primary human hepatocytes (PHH, BioIVT) (1:6 PHH to NPC ratio) in a multi-well 96-well plate format (Elplasia, Corning). We seeded the cells at a density of 40,000 cells/well in William’s E medium supplemented with 11mM glucose, insulin (10ng/mL in Control or 10μg/mL in NASH), 5.5μg/mL transferrin, 6.7ng/mL selenite, 100nM dexamethasone, 2mM L-glutamine, 100U/mL penicillin, 100μg/mL streptomycin, and 10% fetal bovine serum (FBS).
Upon spheroid aggregation (5-7 days), we phased out FBS; to aggravate NASH phenotype we added a 0.6% (w/v) albuminconjugated free fatty acid (FFA) into the media, consisting of equimolar palmitic and oleic acid (240μM final concentration each). FFA was not supplemented in Control, mimicking lifestyle intervention by reducing nutrition and fat uptake. For the treatment groups, we treated the spheroids with FFA and the DCP compounds for 2 weeks with redosing every 2-3 days. We screened 91 probes at their 1x concentration (mostly 1μM, some 0.1μM).
Throughout the DCP screening we identified some DCP hits that reduced significantly at least one of the studied NASH endpoints. We validated the efficacy of these probes by using their non-active analogue at 1x concentration using the same experimental setup as the active compounds, we also tested their dose-dependent effect on the corresponding endpoints by using unchanged experimental setup but testing 0.2x, 1x and 10x of the hit probe.
Read-out:
Inflammation: We collected supernatant after 1 week treatment with DCP compounds to measure pro inflammatory cytokines and chemokines (IL6, IL8 and TNFa) using a Luminex High Performance Assays (FCSTM03-03).
Results
Inflammation: Pro inflammatory cytokines (IL6, TNFa) and the chemokine (IL8) were significantly increased in NASH vs Control group implying the efficacy in using this model to study inflammation as an endpoint of NASH. Some tested probes showed an anti-inflammatory effect by reducing the measured inflammatory markers after 1 week treatment (figure 1).
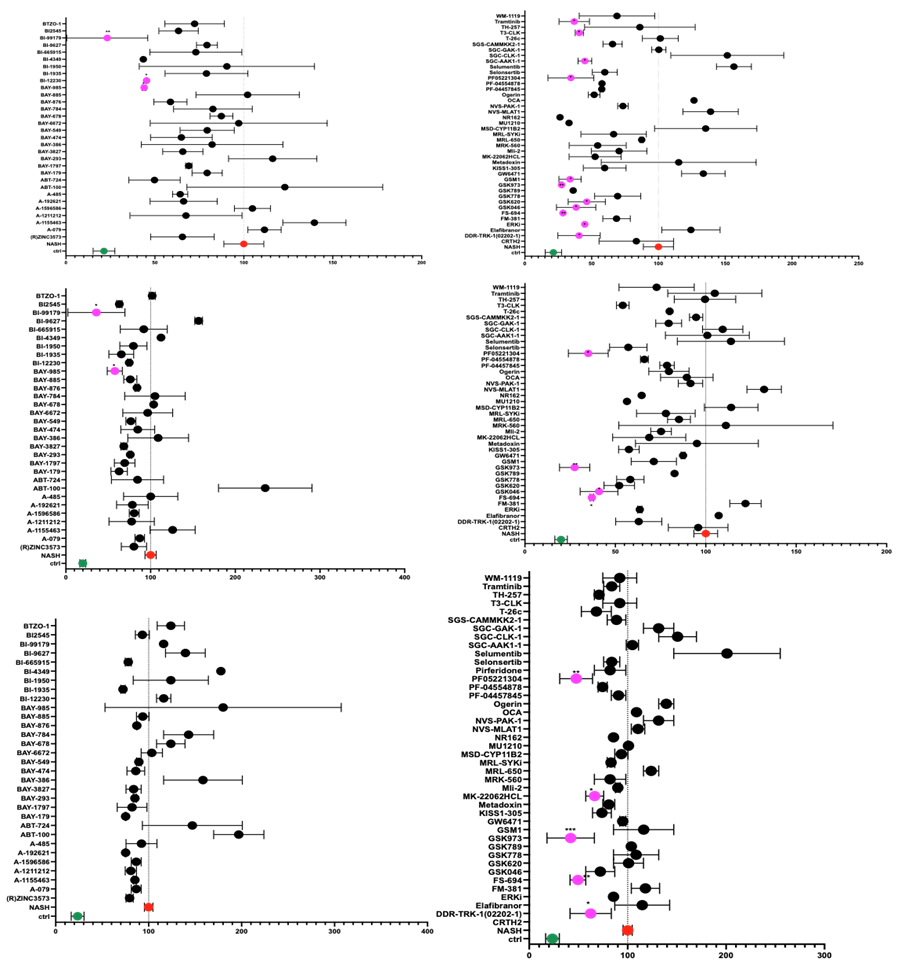
Figure 1: percent cytokine levels in the DCP-treated liver spheroids normalized to NASH in red (100%) and showing the lean group in green. Measurements were done after 1 week of treatment with free fatty acids (FFA) only (NASH), without FFA (lean) or with FFA +DCP. Probe showing significant decrease in cytokine secretion are marked in pink. T-test was performed for statistical analysis using prism. Average values are reported +/- sem with n=2.
Conclusions
We developed a patient-derived assay for NASH that can faithfully reproduce the hallmarks of the disease. We used this assay to screen for potential drug targets using the DCP compound set. As a result of this screening, we have identified probes that efficaciously reduced inflammation revealing potential drug targets for NASH. Additionally, we validated the specificity of the hit probes by using non-active analogues and by showing a dose-dependent effect (data not shown).
References
- Schwabe R et al.; Mechanisms of Fibrosis Development in NASH (2020).
- Kemas et al.; Non-alcoholic fatty liver disease - opportunities for personalized treatment and drug development (2021).



