Aim
To evaluate toxicity of Donated Chemical Probes (DCP) probes in primary human hepatocyte (PHH) spheroid cultures.
Methods
Cell culture conditions: PHH were seeded in ultra-low attachment plate (faCellitate) with density of 1,500 cells per well in William’s E medium (Gibco) supplemented with 2mM L-glutamine, 100U/ml penicillin, 100μg/ml streptomycin, 10μg/ml insulin, 5.5μg/ml transferrin, 6.7ng/ml sodium selenite, 100nM dexamethasone and 10% fetal bovine serum (FBS) [1]. Self-aggregation occurred after a week, and cells were treated for another week with FBS-free media containing DCP compounds with recommended concentrations (1x) and 10-fold higher concentration. Media was replenished at day 3, then cell viability assay was performed at day 7. Cell culture medium without added probes was used as a negative control.
Readout: Cell viability was assessed by cellular ATP assay, CellTiter-Glo Luminescent Cell Viability Assay kit (Promega), with luminescence reading (RLU). Viability was expressed as average RLU from all biological replicates normalized to the average RLU of the negative control. Toxicity was defined as those with normalized ATP significantly <80% of the negative control [2].
Results
At the recommended concentration (1x), 7 out 94 DCP showed hepatotoxicity (8%). At 10-fold higher concentration, however, more than a third (24 compounds) of the DCP was toxic (Figure 1).
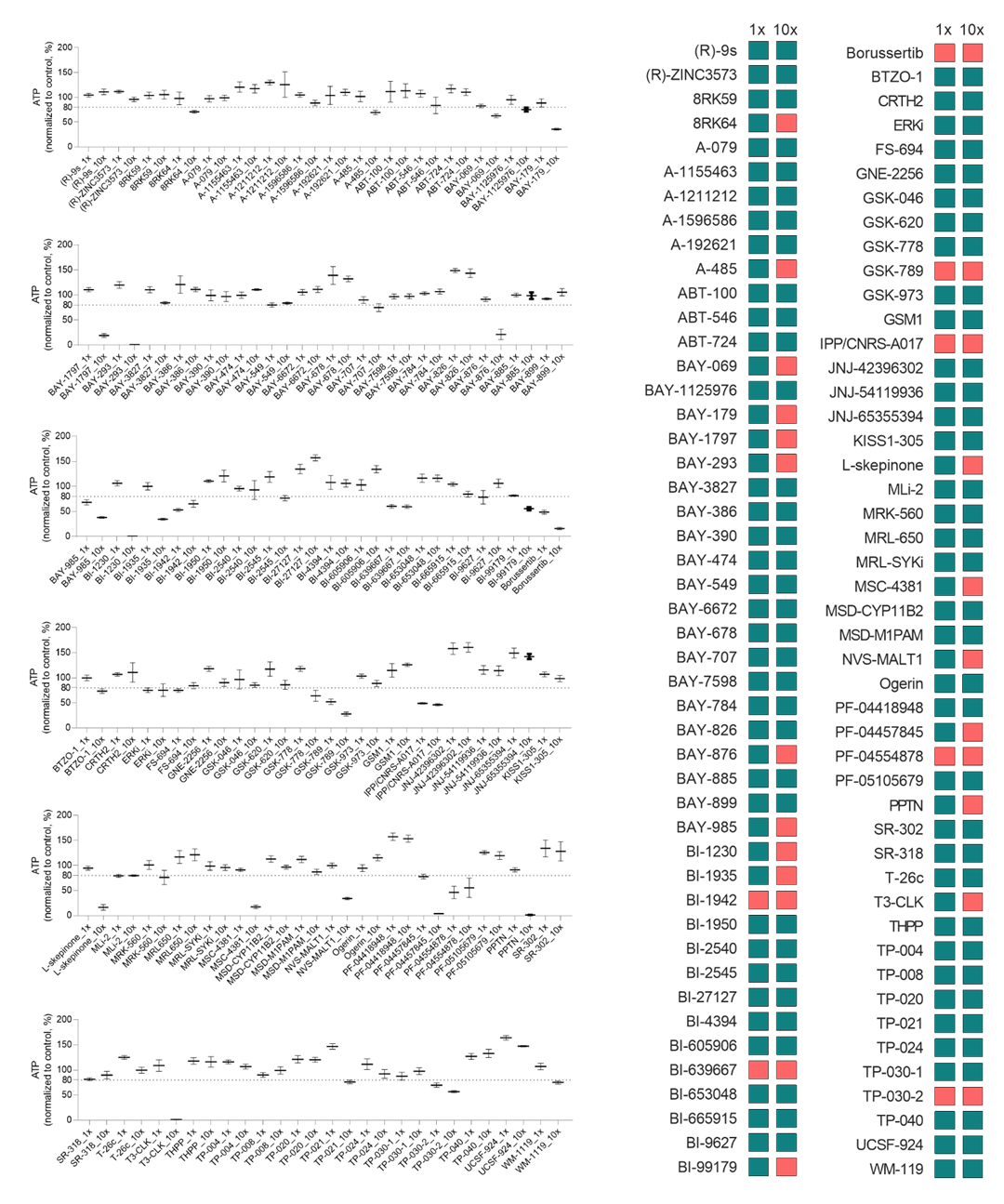
Figure 1. Hepatotoxicity of DCP compounds in 3D PHH spheroids. PHH were treated with each DCP compounds with either recommended dose (1x) or 10-fold higher dose (10x) for 7-day. Left: Normalized ATP expressed in percentage relative to negative control, mean ± s.e.m. (n=4-7 biological replicates). Toxicity is defined as those with cellular ATP significantly less than 80% of the mean ATP of the negative control (p<0.05). Right: Summary of the hepatotoxicity screening of DCP compounds. Red boxes indicate hepatotoxicity at indicated concentrations. Concentrations of the compound in term of molarity can be found in the accompanying Excel file.
Conclusions
Hepatotoxicity of 94 DCP has been evaluated. The results herein can be used as a guidance to determine doses suitable for other in vitro assays and to filter out hits in other tissue assays or endpoints that could be attributed to cellular toxicity.
References
- Bell, C. C. et al. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci. Rep. 6, 25187; doi: 10.1038/srep25187 (2016)
- Vorrink, S.U. et al. Prediction of Drug-Induced Hepatotoxicity Using Long-Term Stable Primary Hepatic 3D Spheroid Cultures in Chemically Defined Conditions, Toxicological Sciences, Volume 163, Issue 2, June 2018, Pages 655- 665, https://doi.org/10.1093/toxsci/kfy058



