Disease area
Inflammatory bowel disease (IBD), comprising Crohn’s disease (CD) and ulcerative colitis (UC), represents a group of chronic inflammatory conditions affecting the gastrointestinal tract. IBD currently impacts approximately 5 million individuals worldwide, with incidence rates rising significantly over recent decades. Despite substantial advances, no definitive cure exists. IBD is characterized by a relapsing-remitting course, and current therapies primarily aim to suppress inflammatory symptoms to maintain patients in remission. However, these treatments often provide limited efficacy, with nearly 50% of patients experiencing relapse or becoming unresponsive over time. Given the complexity and heterogeneity inherent to IBD, there is an urgent need to develop reliable biomarkers and personalized therapies targeting novel mechanisms of disease pathogenesis.
Rationale
Existing biologic therapies, predominantly designed to modulate inflammatory pathways, have shown clinical benefit but fail to achieve sustained therapeutic responses in approximately 35–50% of patients, who either do not respond initially or relapse during treatment. This significant proportion of non-responders highlights the critical need for alternative therapeutic strategies to complement current immunomodulatory interventions. We hypothesize that enhancing mucosal regeneration—an essential determinant associated with decreased hospitalization rates and reduced surgical interventions—could potentially extend remission periods in patients with IBD.
Aim
In the present study, we established an automated medium-throughput screening platform (96-well format) employing human intestinal organoids (HIOs) co-cultured with patient-matched fibroblasts derived from inflamed and non-inflamed biopsies obtained from patients diagnosed with IBD. Our primary objective is to identify drug-like chemical compounds and protein-based reagents capable of promoting intestinal regeneration. Specifically, our approach targets epithelial cells (including stem cells and non-stem niche cells), non-epithelial niche cells (such as fibroblasts), or a combination thereof.
Methods
Biopsies (3–4 per patient) of inflamed colonic mucosa were obtained via colonoscopy from patients diagnosed with IBD. Crypt isolation and subsequent culture of human intestinal cells were performed according to previously established methodologies. (1- 2) Organoids were expanded and maintained in stem cell medium as described in the literature. Culture medium was refreshed every 2–3 days, and organoids were passaged at a ratio of 1:4 approximately every 7–10 days. For three-dimensional (3D) culture, organoids were embedded in 70% Matrigel, diluted in base medium comprising Advanced DMEM F12 supplemented with 100µg/mL gentamicin, 10mM HEPES, and 1X GlutaMAX, and cultured at 37°C, in 5% CO₂.
Residual biopsy tissue remaining after crypt isolation was washed in HBSS and enzymatically digested using a mixture of 1 mg/mL Liberase DH and 0.1 mg/mL DNase I in HBSS at 37°C for 15–20 minutes. Following two rounds of washing with base medium, cells were resuspended in fibroblast culture medium consisting of base medium supplemented with 5ng/mL EGF, 5ng/mL heat-stable bFGF, 0.5μM prednisolone, and 10% charcoal-stripped fetal bovine serum (CSFBS). Cells were initially cultured in Primaria 6-well plates for 3–4 weeks at 37°C, in 5% CO₂. After primary culture, fibroblasts were harvested using 1X TrypLE Express Enzyme and expanded further in Primaria T25 culture flasks using fibroblast medium containing reduced CSFBS concentration (2.5%).
Cultured intestinal organoids were harvested and enzymatically digested with 1X TrypLE Express to obtain single-cell suspensions. Single-cell suspensions of organoids were mixed with patient-matched fibroblasts at a 4:1 ratio in 70% Matrigel diluted with base medium. Mantis microfluidic liquid dispenser was employed to plate cells into 6 replicate 96-well plates for each screening round. Organoid stem cell medium supplemented with the ROCK inhibitor Y-27632 was added using the CyBio SELMA automated pipetting system. Plates were subsequently incubated in an Incucyte S3 live cell analysis instrument at 37°C in 5% CO₂, and images were acquired at 8-hour intervals for a duration of 15 days. After an initial three-day culture period, organoid medium was refreshed, and 96 compounds from the Donated Chemical Probes (DCP) library were added in triplicate using the CyBio SELMA automated pipetting system. Medium containing the compounds was refreshed every 3–4 days.
Read-out: Image analyses were conducted using the Incucyte Organoid Analysis software to measure the growth kinetics of organoids over a 12-day treatment period with chemical probes. Simple linear regression was applied, and the slope was calculated.
Results
To date, the screening platform has been utilized to examine biopsies from three IBD patients with active inflammation, evaluating a total of 96 chemical probes from the Donated Chemical Probes (DCP) collection. Figure 1 presents the slopes of organoids growth kinetics measured over 12 days of chemical probe treatment, normalized relative to the dimethyl sulfoxide (DMSO) control condition, and ranked from highest to lowest growth-promoting effects. Data were derived from three biological replicates corresponding to three individual IBD patients, with each chemical probe treatment performed in triplicate. Several compounds notably enhanced the growth rates of organoids compared to the baseline established by the DMSO-treated controls. Recombinant human amphiregulin (AREG) and Topotecan were used as positive and negative controls respectively.
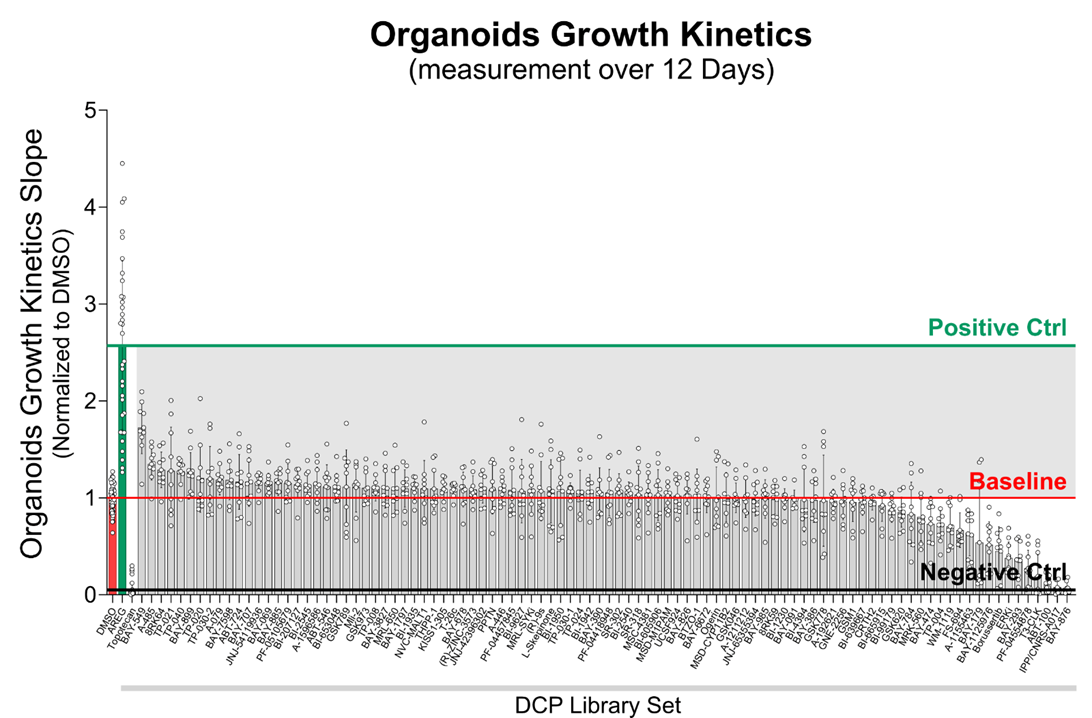
Figure1. Slopes of organoids growth kinetics measured over 12 days of chemical probe treatment, normalized relative to the dimethyl sulfoxide (DMSO) control condition, and ranked from highest to lowest growth-promoting effects. Amphiregulin (AREG) and Topotecan were used as positive and negative controls, respectively. Data were derived from three biological replicates corresponding to three individual IBD patients, with each chemical probe treatment performed in triplicate.
Conclusions
We successfully developed an automated medium-throughput screening platform (96-well format) employing intestinal organoids derived from biopsies of inflamed colonic regions in IBD patients, co-cultured with patient-matched fibroblasts. Screening of a library comprising 96 Donated Chemical Probes (DCP) in this co-culture system facilitated the identification of several promising candidates capable of stimulating regenerative responses. These candidate compounds enhanced the growth kinetics of intestinal organoids derived from IBD patients in the presence of matched fibroblasts, highlighting their potential utility in future therapeutic approaches targeting intestinal regeneration in IBD.
References
- Sato T., et al. , Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology, 2011. 141(5): p. 1762–72.
- Dekkers J.F., et al. , A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med, 2013. 19(7): p. 939–45.
- Borten M.A., et al. , Automated brightfield morphometry of 3D organoid populations by OrganoSeg. Sci Rep, 2018. 8(1): 5319.



