Disease area
Colorectal cancer (CRC) remains one of the most lethal malignancies globally, frequently diagnosed at advanced stages when metastasis has already occurred. Despite significant progress in chemotherapy, immunotherapy, and targeted treatments, resistance to therapy continues to be a major clinical challenge. With the emergence of novel immunotherapies, particularly chimeric antigen receptor T (CAR-T) cell therapy, it is critical to address the underlying resistance mechanisms within the tumor microenvironment to improve patient outcomes.
Rationale
CAR-T cell therapy has achieved groundbreaking success in treating hematological cancers; however, its effectiveness in solid tumors like CRC has been limited. Key obstacles include immune evasion by tumor cells, inhibitory signals within the tumor microenvironment, and insufficient T-cell infiltration. Additionally, intrinsic resistance mechanisms within tumors reduce CAR-T cell cytotoxicity, compromising overall treatment efficacy. To overcome these hurdles, combinatorial strategies incorporating pharmacological modulators are being explored to enhance CAR-T cell function.
Aim
This assay is designed to identify pharmacological modulators that can augment activity CAR-T cells targeted against the tumor antigen CEA (CEACAM5). By screening the Donated Probe Library (DCP), the goal is to discover drugs that enhance tumor cell directed cytotoxicity. Ultimately, this approach seeks to overcome resistance barriers and improve the therapeutic efficacy of CART cell therapy in solid tumors.
Methods
Cell culture: The colorectal cancer (CRC) organoid line O06 was established and maintained according to detailed protocols available on the EUbOPEN website (refer to "Protocol for generation of normal and tumor organoids from human colorectal tissues") and on the recent publication (Farin et al., 2023). CAR-T cells were produced using a 2nd generation CAR against CEA (Hombach et al., 2007). Peripheral blood mononuclear cells (PBMCs) from allogenic healthy donors were freshly isolated, and T cells were lentiviral modified following established procedures.
General Protocol: To assess compound effects in co-culture, organoids were transduced with Luciferase2-P2A-EGFP (Luc2/GFP) lentivirus, as previously described (Schnalzger et al., 2019) (Fig. 1A). 96-well plates were pre-coated with 15 µL of 50% Matrigel, and O06 cells were seeded as single cells onto the Matrigel layer. Organoids were grown for three days in full organoid medium (100 µL/well). On day 4, the medium was removed, and CAR T cells were added along with the DCP library, which included a chemogenomic kinase set consisting of 370 compounds, including negative controls. The screen was performed in two independent replicates.
FACS Analysis: Organoids were singularized and stained with anti-CEA and anti-CD19 antibodies at a 1:200 dilution and life dead staining (eFluor780) at a 1:1000 dilution. Staining was performed for 30 minutes at room temperature, followed by two washes with PBS. Cells were then fixed for 15 minutes at room temperature using 1% PFA in PBS, washed twice, and resuspended in FACS buffer and analyzed on a BD Fortessa instrument.
Readout: On day 7, viability was measured using the ONE-Glo™ EX Luciferase Assay System (E2920, Promega) according to the manufacturer’s instructions.
Results
CEA surface expression was analyzed on CRC organoids (O06) by flow cytometry (Fig. 1A/B). Robust CEA expression was detected and O06 was negative for CD19 expression (detected by control CD19-targeted CAR-T cells). In a subsequent cytotoxicity assay, O06 organoids were co-cultured with CAR-T cells (Fig. 1C), which showed efficient killing by CEA-CAR T cells, whereas off-target CD19 CAR-T cells exhibited no cytotoxic activity, confirming the antigen specificity (Fig. 1D). Various CAR-T cell numbers were assessed to determine the optimal effector-to-target (E:T) ratio for subsequent pharmacologic screening. A lysis rate of approximately 30–40% was achieved using 300 CAR T cells, to provide a dynamic range for subsequent compound modulation studies (Fig. 1E). To study if CAR-T cell activity can be further enhanced by pharmacological modulators, we conducted a drug library screen. This library comprised 262 active compounds and 108 negative controls, all of which had been previously characterized by the Donated Chemical Probes (DCP) and Structural Genomics Consortium (SGC) programs or assembled as part of the EUbOPEN chemogenomic libraries. The compound screen was conducted in duplicate wells and at a single concentration. Each drug was tested in organoids alone (O06) and in a co-culture with CEA CAR T (Fig. 2A). The duplicate viability data demonstrated robustness both in mono-culture and co-culture conditions, with a good correlation between replicates (Fig. 2B). This combined approach enabled the identification of compounds that specifically increased the killing efficiency of organoids by CAR T cells, but did not affect the viability of organoids alone (Fig. 2C-D).
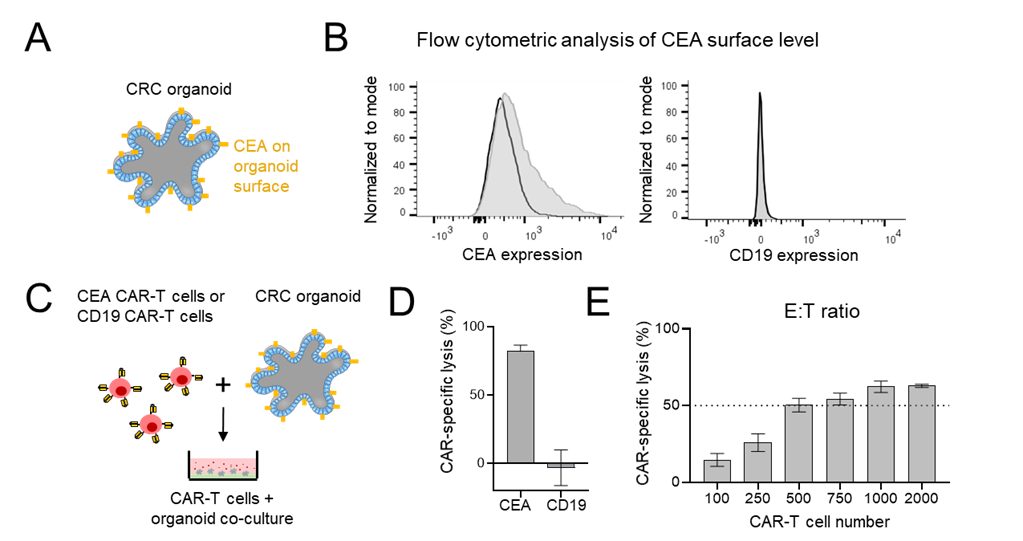
Figure 1. CEA CAR T cell assay to study cytotoxicity against CRC PDTOs. (A) Schematic depiction of colorectal cancer patient-derived tumor organoids (PDTOs) with surface expression of carcinoembryonic antigen (CEA). (B) Flow cytometric analysis of surface markers in PDTO line O06 stained with anti-CEA (left) and anti-CD19 (right). A 27% shift in fluorescence intensity compared to isotype control staining indicates positive CEA expression, while CD19 staining serves as a negative control. (C) Experimental setup for assessing CAR-T cell killing efficiency in a 96 well format. Organoids were co-cultured with CEA or CD19 targeted CAR-T cells. (D) Quantification of cytotoxicity after 3 days in presence of 3,000 CAR-T cells per well. Target cell killing was measured by luciferase-based assay and displayed as a bar graph. Error bars represent standard deviations from four technical replicates. (E) Cytotoxicity at varying effector-to-target (E:T) ratios, measured by the same luciferase-based method.
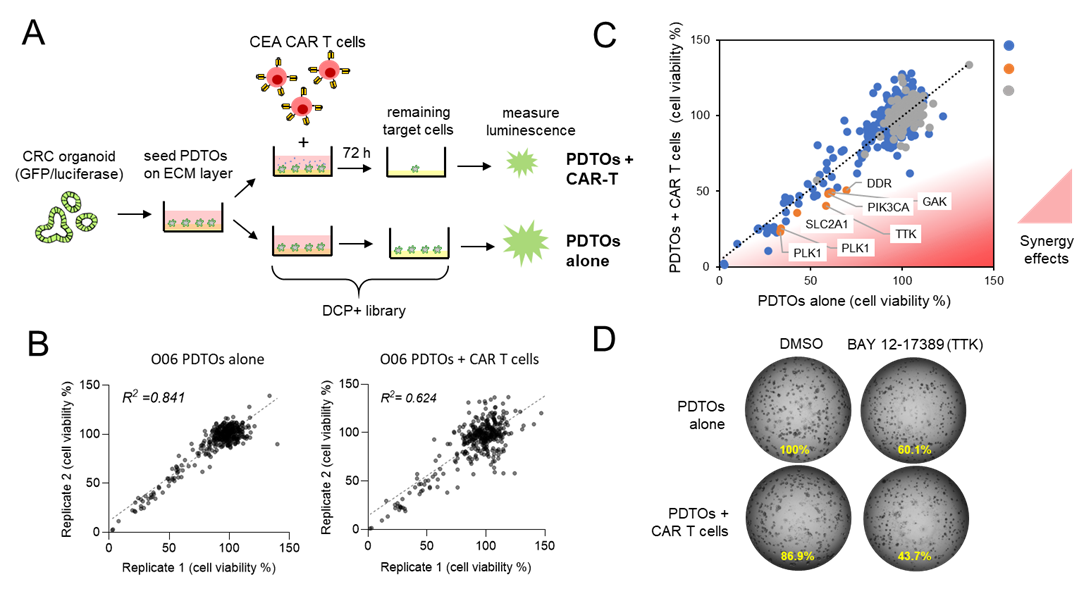
Figure 2: Pharmacologic synergy screen to enhance CAR-T cell efficiency against CRC organoids. (A) Schematic representation of the experimental workflow. CRC patient-derived tumor organoids (O06) were cultured alone or with a limiting number of CEA CAR-T cells. The DCP+ pharmacologic compound library was tested in both conditions to assess synergistic effects on cytotoxicity. (B) Correlation analysis of screening replicates to study assay robustness. Scatter plots depict the correlation between two independent replicates for the entire screening dataset in both mono-culture (left) and co-culture (right) conditions. (C) Pharmacologic screen reveals treatment-specific resistance mechanisms. A library of 370 compounds, including negative controls, was tested in O06 under both mono-culture and co-culture conditions with CEA CAR-T cells. Viability was measured via luciferase assay. Co-culture-induced synergy was assessed by comparing tumor cell viability in organoids alone versus organoids co-cultured with CAR-T cells (red area). Top hits are highlighted in orange. (D) Example images of PDTOs after 3 days of mono and co-culture in presence of DMSO (control) of the TTK inhibitor (BAY 12-17389).
Conclusions
Our work underscores the potential of CRC organoids as a reliable model for exploring novel therapeutic approaches in solid tumors. The integration of pharmacological modulators with CAR-T cell therapy represents a promising strategy to counteract the resistance mechanisms observed in CRC. This approach facilitates the discovery of drugs that can enhance immune cell efficacy in specific manner. Our screening platform provides a foundation for advancing personalized immunotherapies and offers mechanistic insights that could allow more effective CAR-T cell therapies, ultimately improving clinical outcomes for patients with solid malignancies.
References
- Farin HF, Mosa MH, Ndreshkjana B, et al. Colorectal Cancer Organoid-Stroma Biobank Allows Subtype-Specific Assessment of Individualized Therapy Responses. Cancer Discov. 2023;13(10):2192-2211. doi:10.1158/2159-8290.CD-23- 0050
- Hombach AA, Schildgen V, Heuser C, Finnern R, Gilham DE, Abken H. T cell activation by antibody-like immunoreceptors: the position of the binding epitope within the target molecule determines the efficiency of activation of redirected T cells. J Immunol. 2007;178(7):4650-4657. doi:10.4049/jimmunol.178.7.4650
- Schnalzger TE, de Groot MH, Zhang C, et al. 3D model for CAR-mediated cytotoxicity using patient-derived colorectal cancer organoids. EMBO J. 2019;38(12):e100928. doi:10.15252/embj.2018100928



