Disease area
Colorectal cancer (CRC) remains one of the most frequent neoplasms and is considered the second leading cause of cancer death worldwide. Surgery and chemotherapy have improved the prognosis for many CRC patients. However, frequent relapse in patients with metastatic disease results in only 12% survival after 5 years, highlighting an urgent need for alternative treatment approaches. Because CRC is characterized by prominent genetic and phenotypic heterogeneity between patients the identification of actionable drivers for personalized therapy remains challenging.
Rationale
Patient-derived tumor organoids have recently emerged as preclinical models that faithfully recapitulate the molecular and phenotypic characteristics of CRC. 3D organoids can be obtained from primary tumor material and adjacent healthy tissue. Organoid biobanks have been used to identify patient-specific vulnerabilities. Drug testing could thereby allow to identify common and patient-specific resistance and sensitivity phenotypes.
Aim
Cell viability screening matched tumor and healthy colorectal patient-derived organoids was performed to investigate the toxicity of the donated chemical probes (DCP).
Methods
Cell culture condition: PDOs were established and cultured as previously described (van de Wetering et al., 2015). For detailed information on organoid handling and culturing refer SOP. Tumor and normal cells are cultured in 100 µl/well medium containing advanced DMEM/F12 supplemented with 10 mM Hepes, 1× Glutamax, 1× penicillin/streptomycin, 2% B27, 12.5 mM Nacetylcysteine, 500 nM A83-01, 10 μM SB202190, 20% R-spondin 1 conditioned medium, 10% Noggin conditioned medium, 50 ng/ml human EGF and 35 ng/ml Wnt surrogate.
General protocol: Colony formation assay was performed for each tumor-normal pair to standardize the input organoid number for compound screen. Tumor and normal organoids were enzymatically dissociated, seeded in 15 µl 90% Matrigel in 96-well round bottom plates in duplicate plates and let recover for 3 days before exposure to chemical probes for 4 days. For the validation of the hit compounds tumor and normal organoids (n=3) were seeded in triplicate as described above. Chemical probes were dissolved in DMSO at: 1, 2 or 10 mM and further diluted (10.000x) to a final concentration of 0.1, 0.2 or 1 µM. For validation, compounds were automatically dispensed by Tecan D300e Digital Dispenser in logarithmic 7-point dilutions concentration ranging from 0.01-10 µM.
Readout: Viability of cells was measured by CellTiter-Glo assay after 7 days of culture. Raw data of initial screen was averaged from duplicate plates and from triplicates for hit validation. The average sensitivity was used to score for hits.
Results
A set of 192 chemical compounds (including controls) was tested in 5 patient-derived colorectal organoids. At a 75% threshold, 7 and 10 hits were identified in T and N organoids, respectively, of which six DCP probes were shared between tumor and normal entities. Numerous hit compounds showed a similar effect across all patients, yet we also observed patient-specific sensitivities. To identify compounds that specifically target tumor cells, the T/N ratio was calculated, which revealed 3 tumor-selective compounds. 3 compounds showed stronger effects in normal cells. We noticed a T/N ratio of almost 40-fold for the BCL-XL inhibitor A-1155463 (Figure 1).
We validated the top hits by dose-titration experiments. A pronounced toxicity was observed in tumor organoids for the BCL-XL inhibitor A-1155463 with IC50 between 0.02-0.1 μM that caused no toxicity in normal lines at comparable concentrations. Inhibition of the related protein BCL-2, using A-1211212 probe, neither affected T nor N cells, demonstrating a highly specific vulnerability of CRC cells to BCL-XL inhibition (Figure 2).
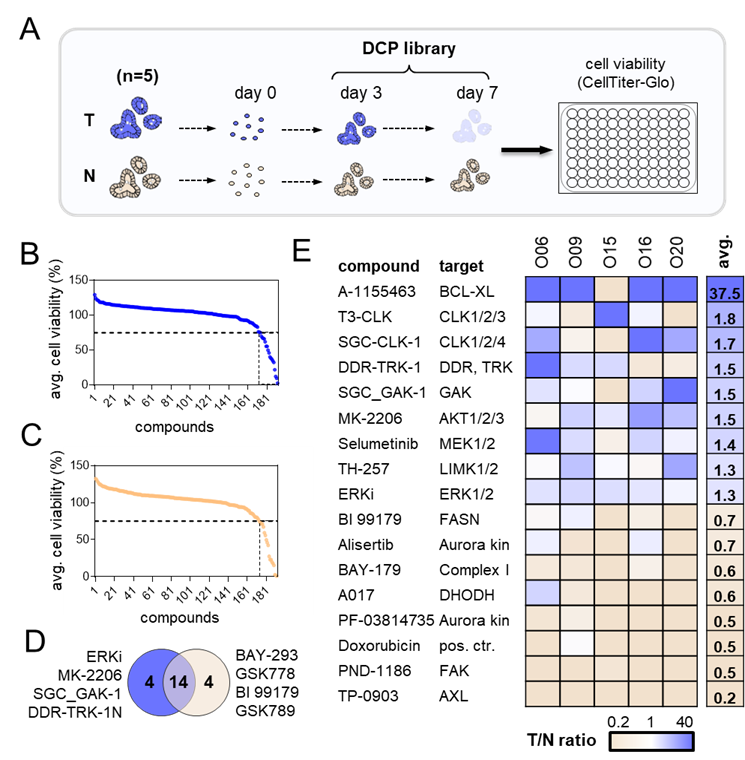
Figure 1. DCP library screen in matched tumor and normal organoids. (A) Schematic workflow. (B/C) Average cell viability of all tumor lines (B) and normal lines (C). 75% cell viability cut-off is applied for hit identification. (D) Venn diagram showing the overlap between top hits in tumor and normal. Four specific cytotoxic compounds are observed in each. (E) Ratio of cell viability in tumor vs normal is represented in the heatmap, where blue indicates strong compound effects in tumor organoids and orange shows strong effects in normal lines.
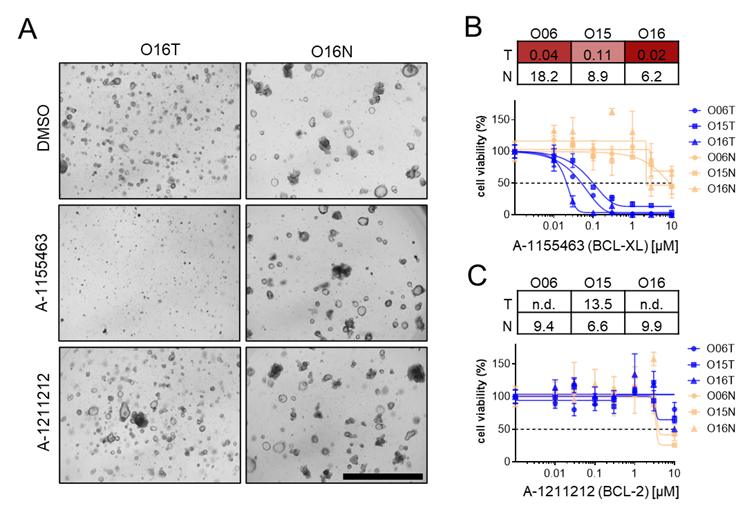
Figure 2. Validation of hit compounds. (A-C) Tumor-selective sensitivity to BCL-XL inhibitor A-1155463. (A) Morphological images (O16) after 4-day treatment with 1 µM A-1155463 or the BCL-2 inhibitor A-1211212. (B/C) dose-response curves of cell viability in tumor and normal organoids (blue and orange curves, respectively).
Conclusions
Our analysis revealed common tumor-specific vulnerabilities, indicating possibilities for personalized therapy targeting known and previously uncharacterized targets in CRC. The data has been published in the following paper „Deep Annotation of Donated Chemical Probes (DCP) in Organotypic Human Liver Cultures and Patient-Derived Organoids from Tumor and Normal Colorectum “. Tredup C., Ndreshkjana B., et. al., ACS Chemical Biology 2023.
References
- Deep Annotation of Donated Chemical Probes (DCP) in Organotypic Human Liver Cultures and Patient-Derived Organoids from Tumor and Normal Colorectum “. Tredup C., Ndreshkjana B., et. al., ACS Chemical Biology 2023.



